Research
Project Outline
Hematopoiesis is one of stem cell-sustained somatic organs in which billion of blood cells are being formed every day throughout lifetime of an individual. Blood homeostasis is maintained by a very rare population of hematopoietc stem cells (HSCs) that slowly self-renew and multi-lineage differentiate into functionally mature blood cells via rapidly dividing lineage committed intermediate progenitors. Such high regenerative capacity of HSC is conserved in a specialized microenvironment in the bone marrow (BM), often referred to as "BM niche" that supplies pivotal factors for HSC maintenance.
Our projects to understand physiology and pathophysiology of hematopoietic stem cells are subdivided into the following three specific aims:
01
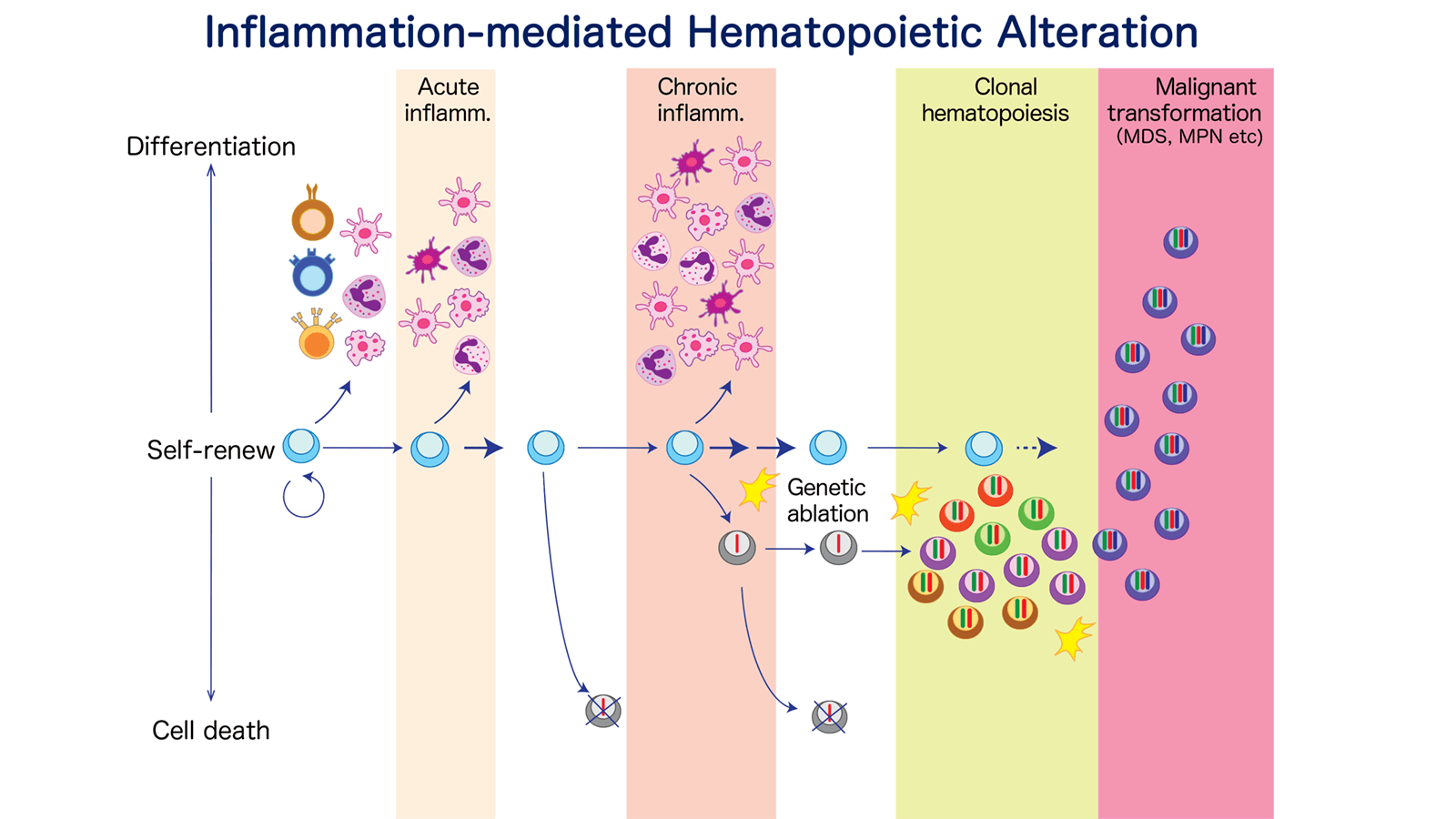
Understanding of ageing-associated functional changes in HSC and their-derived hemato-immune system
Ageing of hemato-lymphoid system is characterized by thymic involution, expansion of memory B and T cell pool, narrowed spectrum of acquired immune receptors, favored myelopoiesis at the expense of lymphopoiesis, clonal hematopoiesis, all of which together lead to increased susceptibility to infection, and increased risk of myeloid blood cancer. Some of these ageing phenotypes are attributed from functional changes of HSCs, including decreased self-renewal, myeloid biased lineage output, and accumulation of genetic ablations. Here we set out to ask the questions, 1) how relatively intrinsic and extrinsic changes affect HSC functionality during ageing, e.g., clonal hematopoiesis, and 2) what is the biological impact of aged HSC-derived alterations of hemato-immune system on other organ homeostasis, mainly focusing on HSC competitive fitness and their-derived inflammatory signals.
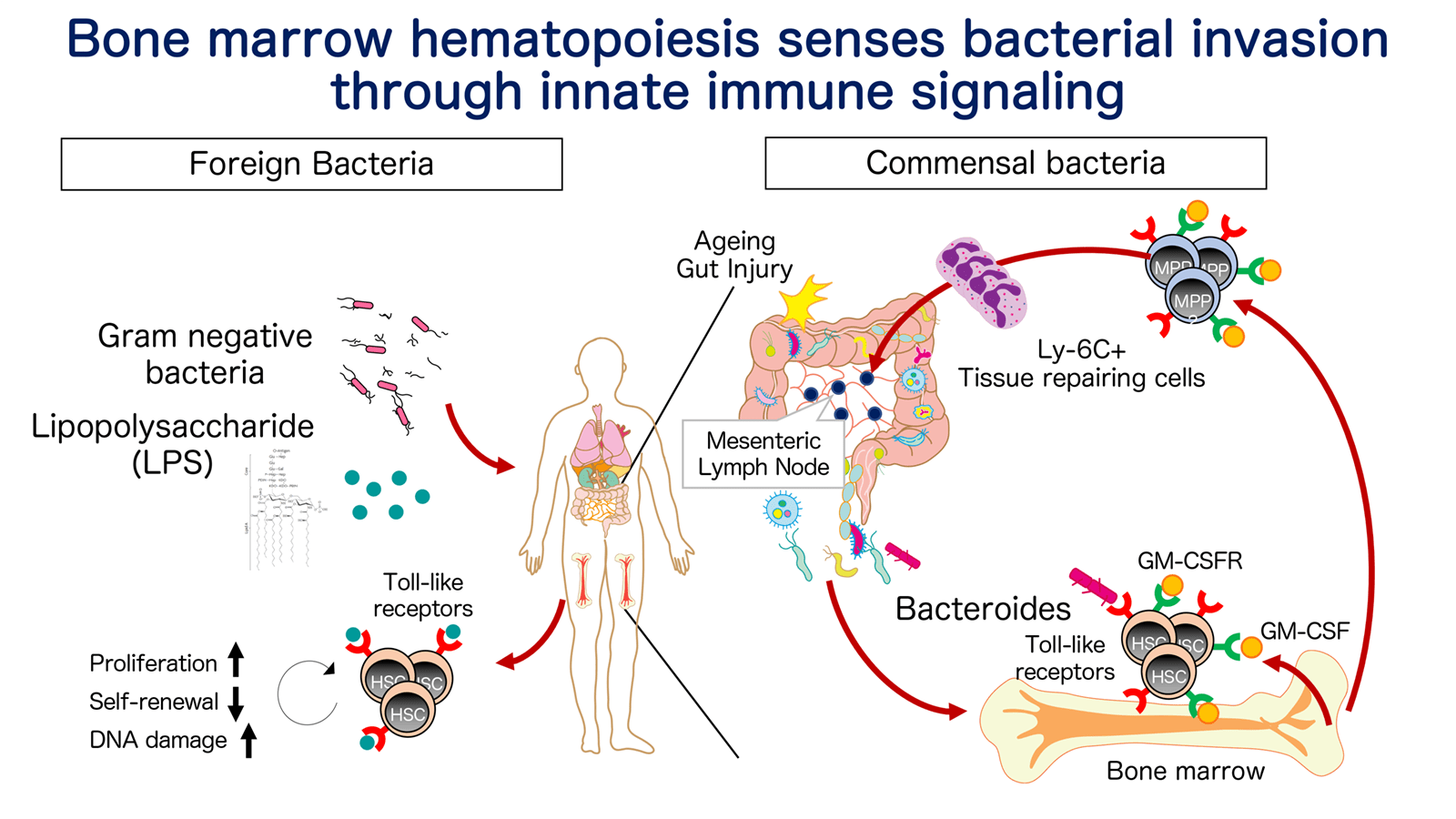
02
Innate immune signaling-mediated sensing mechanism in HSC/progenitor
Upon hematopoietic challenges such as infection, inflammation, short-lived immune effector cells are rapidly consumed at the site of hematopoietic demand, and need to be replenished by HSCs in bone marrow (BM) eventually. Fundamental questions are 1) how the hematopoietic demand in peripheral tissue is sensed and translated to hematopoiesis by distal BM-resident HSC, 2) if HSC/progenitor can retain the adaptation program and fine-tune hematopoiesis for future inflammation. In this project, we aim to tackle this question by using mouse models in which acute or chronic inflammation is induced upon bacterial infection, microbial infiltration, autoimmunity and so on. Systemic injection of lipopolysaccharide (LPS, a ligand for Toll-like receptor (TLR)-4), recapitulating bacterial infection, has demonstrated that LPS directly activates TLR4 in HSCs and decreased their competitive fitness through increased division and DNA damage responses (Takizawa et al, Cell Stem Cell 2017). Bacteria also co-exist with our body and constitute commensal microbe. Upon acute colitis allowing microbial infiltration, a specific type of microbial species activate BM hematopoiesis through TLRs and direct hematopoietic progenitors into inflamed lymph node in which they generate anti-inflammatory effector cells to dissolve inflammation and enhance tissue repair (Sezaki et al., EMBO 2022). Given microbial invasion can occur not only upon gut damage but also under relatively physiological situations such as ageing, these findings indicate pathogen recognition receptor-mediated demand-adapted program in HSC, and propose possibility that chronic inflammation triggered by tissue damage, ageing, increases risk for development of clonal hematopoiesis, accumulation of genetic ablations, and malignant transformation, as observed in the population-based studies and animal models. We have extended these findings to study how inflamed HSC influences lifelong hemato-immune function.
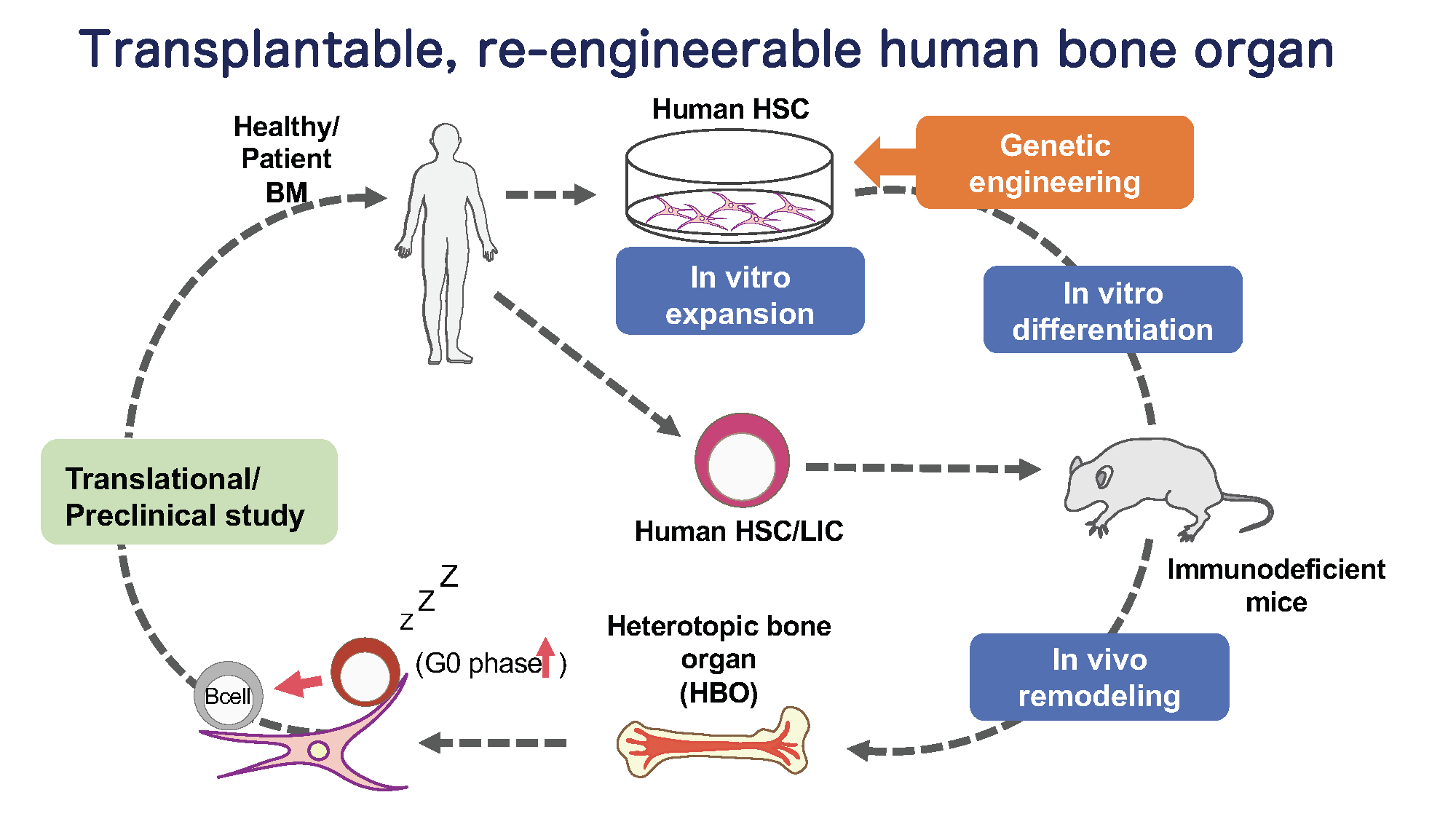
03
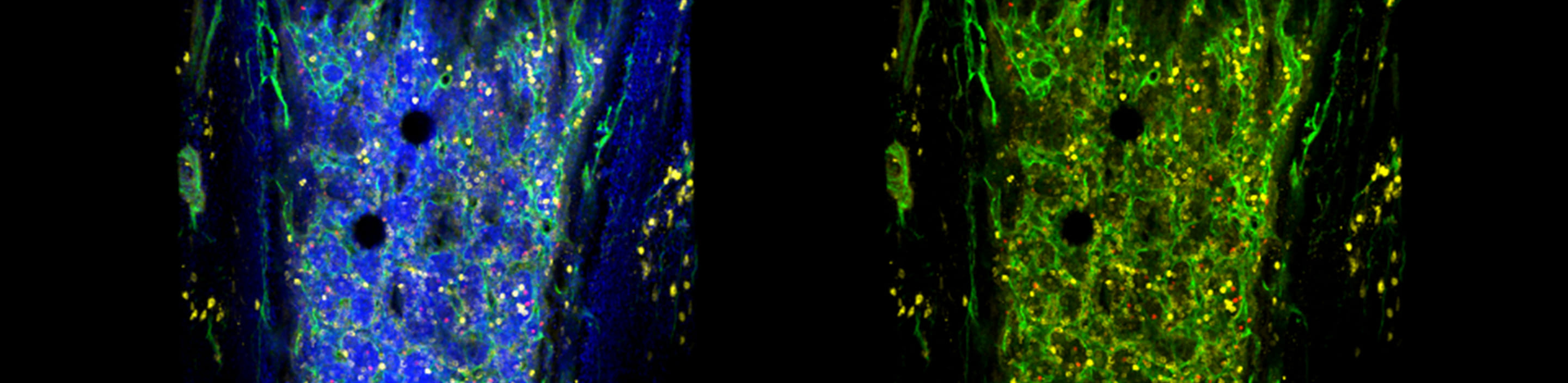
Studying human HSC and niche biology by using a novel humanized mouse model with functional human bone organ
While genetic engineering of animal models have allowed to uncover many vital factors that regulate self-renewal and differentiation of HSCs, little is known about the cellular and molecular components of the human BM niche.
In this project, we take a developmental tissue engineering approach that allows to ex vivo generate a human cartilage template (ossicle) with human adult BM-derived mesenchymal stromal cells (MSCs), and to in vivo develop functional human bone organs capable of human HSC quiescence (Scotti, Piccinini, Takizawa, et. al., PNAS 2013; Fritsch, et al., Exp. Hematol., 2018). To study human BM niche in steady-state and during hematopoietic regeneration, we aim to generate the ossicles derived from normal human BM-MSCs, and dissect cellular and molecular BM niche components by reengineering the ossicles via genetic manipulation of MSCs. This transplantable, re-engineerable human bone organ unit will serve a versatile platform not only to understand human BM niche homeostasis and disease, but also to preclinically test newly-developed drugs or develop new therapy for blood cancer.